Real-Time Monitoring of the Antiseizure Drug Valproic Acid Using a Novel Point-Of-Care Mass Spectrometry
Aims: To improve the timeliness and accuracy of anti-epilepsy drug monitoring, we developed a point-of-care mass spectrometry (PoC MS) for real-time quantification of valproic acid (VPA), as a paradigm for optimizing perioperative management.
Methods: The PoC MS integrates a miniature mass spectrometer, paper capillary spray, and selective ion isolation. A total of 119 blood samples were analyzed for VPA quantification. An equivalent calibration model was established with 12 paired serum and whole blood samples. Analytical performance was benchmarked against liquid chromatography-mass spectrometry (LC–MS) and enzyme multiplied immunoassay technique (EMIT) with 50 whole blood samples. A validation cohort of 45 samples collected at different time points from 9 patients was analyzed to monitor perioperative VPA concentration dynamics.
Results: Selective ion isolation could improve signal intensity by threefold and enable VPA quantification down to 10μg/mL
within 3min, demonstrating a clear speed advantage over other methods. The calibration model showed excellent agreement
between whole blood and serum concentrations (R2=0.978). PoC MS measurements of VPA closely matched LC–MS (r=0.990, bias=0.16%) and EMIT (r=0.988, bias=5.4%). In clinic, VPA level varied by up to 15-fold across patients, and bedside PoC MS could depict dynamic VPA concentration profiles, with intra-patient fluctuations averaging 26%, thereby supporting drug monitoring and therapeutic decision-making.
Conclusion: PoC MS could enable rapid, accurate, and matrix-tolerant bedside monitoring of VPA from whole blood, supporting personalized antiseizure therapy and real-time clinical decision.
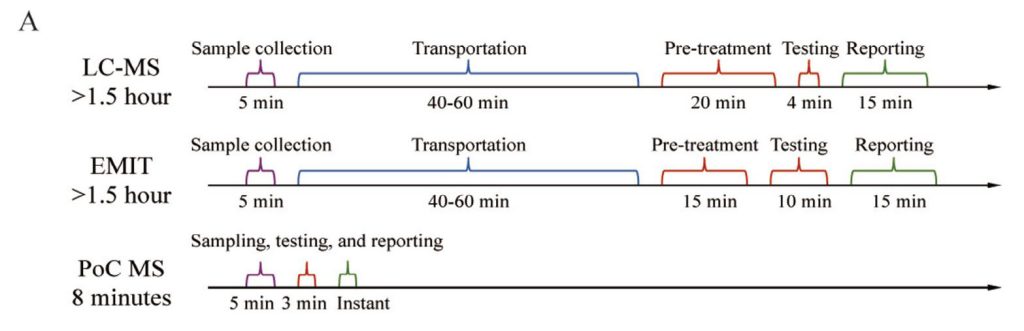
This study introduces a well-designed PoC MS system for realtime VPA monitoring in neurosurgical patients, enabling rapid and accurate quantification directly from whole blood. The system demonstrated performance comparable to conventional methods while significantly reducing turnaround time, thereby facilitating personalized clinical management.
CNS Neuroscience & Therapeutics, 2025; 31:e70499


