Profiling of Cholesteryl Esters by Coupling Charge-Tagging Paternò–Büchi Reaction and Liquid Chromatography–Mass Spectrometry
The profile of cholesteryl esters (CEs) is increasingly used in metabolic disease monitoring due to the roles of CE in regulating the cholesterol level. While electrospray ionization–tandem mass spectrometry is routinely applied for the identification and quantitation of CE, it has a limitation of not being able to provide the location of carbon–carbon double bond (C=C) within unsaturated fatty acyls. In this study, we paired offline 2-acetylpyridine (2-AP) Paternò–Büchi (PB) reaction and reversed-phase liquid chromatography–tandem mass spectrometry to achieve highly sensitive and structural informative CE analysis from complex mixtures. The 2-AP PB reactions of CE standards provided 20–30% conversion but resulted in enhanced ion signal relative to that of intact CE detected as ammonium adduct ions. MS/MS of 2-AP derivatized CE via collision-induced dissociation produced two abundant diagnostic ions for each C=C in a fatty acyl, leading to both sensitive identification and quantitation of C=C location isomers. Twelve saturated and twenty-seven unsaturated CEs were profiled in pooled human plasma; of the latter group, relative quantitation of 6 groups of C=C location isomers was achieved. A dehydrocholesteryl ester, DHE 18:2 (Δ9,12), was confidently differentiated from coexisting compositional isomers: CE 18:3 (Δ9,12,15) and CE 18:3 (Δ6,9,12). The above results represented improved CE coverage at the C=C location level over those reported by gas chromatography MS or acetone PB-MS/MS methods.
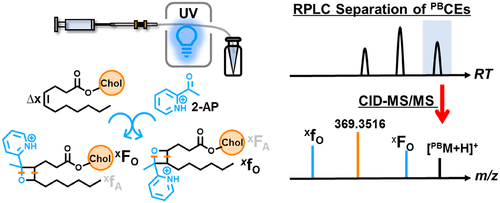
- This study describes a highly sensitive and structurally informative method for cholesteryl esters (CEs) analysis by combining the 2-acetylpyridine Paternò-Büchi reaction with reversed-phase liquid chromatography-tandem mass spectrometry (RPLC-MS/MS).
- The method enables the reliable identification and relative quantitation of C=C location isomers of CE species in human plasma, even including co-existing dehydrocholesteryl ester species.
Anal Chem. 2020 Jun 16;92(12):8487-8496. (IF: 6.7)


