Evaluation of a miniature mass spectrometer based point-of-care-test method for direct analysis of amlodipine and benazepril in whole blood
A miniature mass spectrometer (mMS) based point-of-care testing (POCT) method was evaluated for on-site detecting the hypertension drugs, amlodipine and benazepril. The instrument parameters, including voltage, ISO1, ISO2, and CID, were optimized, under which the target compounds could be well detected in MS2. When these two drugs were injected simultaneously, the mutual ionization inhibition and mutual reduction between amlodipine and benazepril were evaluated. This phenomenon was severe on the precursor ions but had a small impact on the product ions, thus making this POCT method suitable for analysis using product ions. Finally, the method was validated and applied. The blood samples from patients were tested one hour after oral administration of the drugs (20 mg), and the benazepril was quantitatively analyzed using a standard curve, with detected concentrations ranging from 190.6 to 210 μg L-1 and a relative standard deviation (RSD) of 8.6 %. In summary, amlodipine has low sensitivity and can only be detected at higher concentrations, while benazepril has high sensitivity, good linearity, and even meets semi-quantitative requirements. The research results of this study are of great clinical significance for monitoring blood drug concentrations during hypertension medication, predicting drug efficacy, and customizing individualized medication plans.
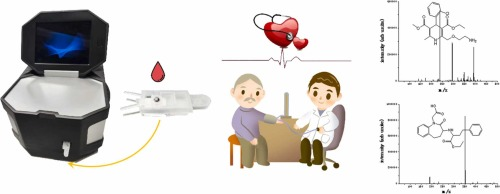
A mMS based POCT method for the rapid on-site screening of hypertension drugs, amlodipine and benazepril, was evaluated. The parameters of this method, such as voltage, ISO1, ISO2, and CID, were determined for amlodipine and benazepril. The method was validated and applied to blood samples from patients who had received oral administration of the drugs. Amlodipine was not detected, indicating low sensitivity at lower concentrations. Conversely, benazepril exhibited high sensitivity, good linearity, and fulfilled the semi-quantitative requirements. This study provides doctors with a rapid, accurate, and personalized drug monitoring method, aiding in understanding the concentration levels of drugs within patients’ bodies, thus enabling better adjustment of drug dosage and enhancing patient treatment outcomes. Based on monitoring results, doctors can devise personalized treatment plans, taking into account individual variation in patients’ responses and tolerances to drugs, thereby providing treatment approaches more tailored to patients’ needs. Additionally, it helps doctors promptly identify instances of drug overdose or underdose, thereby reducing adverse reactions and occurrences of drug side effects, enhancing treatment safety, and providing a better basis for treatment decisions for the medical team.
Journal of Pharmaceutical and Biomedical Analysis 245 (2024) 116194,