Deep Profiling of Aminophospholipids Reveals a Dysregulated Desaturation Pattern in Breast Cancer Cell Lines
Phosphatidylethanolamines (PEs), ether-PEs, and phosphatidylserines (PSs) are glycerophospholipids harboring a primary amino group in their headgroups. They are key components of mammalian cell membranes and play pivotal roles in cell signaling and apoptosis. In this study, a liquid chromatography–mass spectrometry (LC–MS) workflow for deep profiling of PEs, ether-PEs, and PSs has been developed by integrating two orthogonal derivatizations: (1) derivatization of the primary amino group by 4-trimethylammoniumbutyryl-N-hydroxysuccinimide (TMAB-NHS) for enhanced LC separation and MS detection and (2) the Paternò–Büchi (PB) reaction for carbon–carbon double bond (C═C) derivatization and localization. Significant improvement of the limit of identification down to the C═C location has been achieved for the standards of PSs (3 nM) and ether-PEs (20 nM). This workflow facilitates an identification of more than 200 molecular species of aminophospholipids in the porcine brain, two times more than those identified without TMAB-NHS derivatization. Importantly, we discovered that the n-10 isomers in C16:1 and C18:1 of aminophospholipids showed elevated contribution among other isomers, which correlated well with an increased transcription of the corresponding desaturase (FADS2) in the human breast cancer cell line (MDA-MB-231) relative to that in the normal cell line (HMEC). The abovementioned data suggest that lipid reprograming via forming different C═C location isomers might be an alternative mechanism in cancer cells.
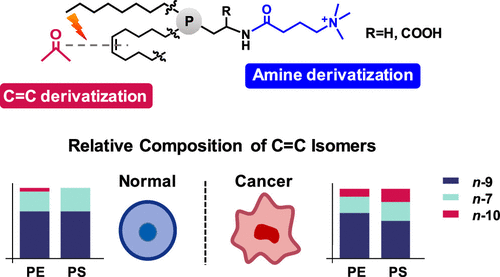
- This study presents a liquid chromatography-mass spectrometry (LC-MS) workflow for sensitive and in-depth profiling of aminophospholipids by integrating two orthogonal derivatization strategies: amine derivatization with 4-trimethylammoniumbutyryl-N-hydroxysuccinimide (TMAB-NHS) to enhance LC separation and MS detection, and C=C derivatization via the Paternò-Büchi (PB) reaction for C=C location analysis.
- It is found that elevated n-10 C=C location isomers in C16:1 and C18:1 of aminophospholipids in the human breast cancer cell line are correlated with increased transcription of FADS2 desaturase, uncovering lipid reprogramming based on alternative desaturation in cancer cells.
Anal Chem. 2022 Jan 18;94(2):820-828. (IF: 6.7)


