Characterization of Fatty Acyl Modifications in Phosphatidylcholines and Lysophosphatidylcholines via Radical-Directed Dissociation
Phosphatidylcholines (PCs) are the major structural components of the plasma membrane of mammalian cells, while lysophosphatidylcholines (LPCs) are critical intermediates in lipid remodeling. Conventional tandem mass spectrometric (MSn) methods via collision-induced dissociation (CID) are blind to intrachain modifications such as the location of the carbon–carbon double bond (C═C) and methyl branching point. In this study, we demonstrate that almost complete structural information can be inferred from a single MS2 CID spectrum of the bicarbonate anion adducts of PC or LPC ([M + HCO3]−), including the identity of the headgroup, composition of fatty acyl chains, their sn-positions, the location of C═C, and the point of methyl branching in fatty acyls. We have integrated this MS2 CID method onto liquid chromatography for the analysis LPCs in human plasma, revealing the existence of multiple sn-isomers, branched chain isomers, and C═C location isomers of LPC.
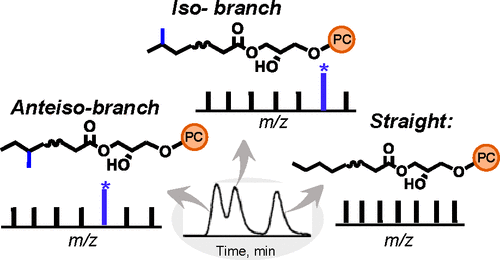
- This study develops a tandem mass spectrometry (MS2) method based on collision-induced dissociation (CID) of bicarbonate anion adducts to achieve comprehensive structural characterization of phosphatidylcholines (PCs) and lysophosphatidylcholines (LPCs).
- The method enables simultaneous identification of headgroup type, fatty acyl composition, sn-position, C=C location, and methyl branching from a single MS² CID spectrum.
- Analysis of human plasma LPCs reveals the presence of multiple sn- isomers, branched-chain isomers, and C=C location isomers, highlighting the power of the method for lipid structural isomer profiling.
J Am Soc Mass Spectrom. 2021 Feb 3;32(2):560-568. (IF: 3.1)


