Organic Chemistry Teaching Experiment - Compound Characterization
In the experiment of “Synthesis and characterization of cinnamic acid,” cinnamic acid is synthesized using benzaldehyde and acetic anhydride through the Perkin reaction under alkaline conditions. Then, unreacted benzaldehyde is removed using steam distillation, followed by decolorization, crystallization, and recrystallization to obtain the final product cinnamic acid, which is then characterized. In the “Continuous extraction method for caffeine extraction” experiment, we use a Soxhlet extractor for continuous extraction. After the concentration and sublimation steps, we finally obtained caffeine and then characterized it. This experiment can help students better understand the extraction and purification process of natural products.
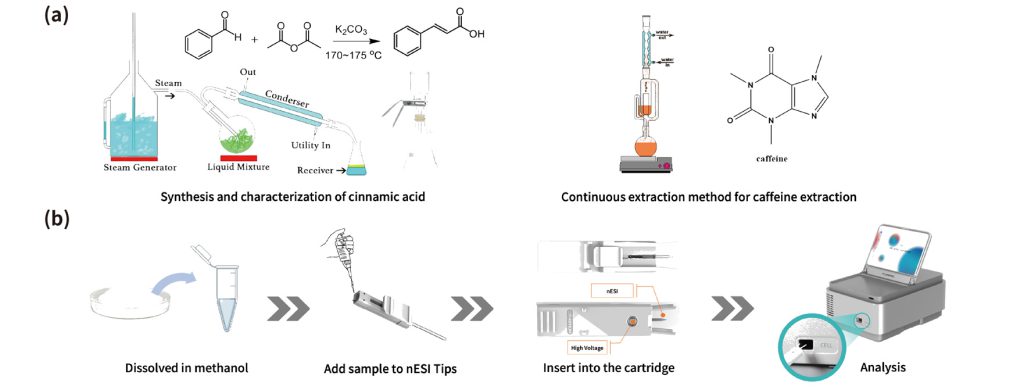
Figure 1. Organic experiments and testing processes.(a) Organic experimental process of cinnamic acid and caffeine.
(b) Detection process of cinnamic acid and caffeine.
Using a miniature mass spectrometer for product characterization mainly involves weighing and dissolving the synthesized or extracted final product, adding the test solution to the nESI Tips, then install the nESI Tips to the cartridge and inserting it into the miniature mass spectrometer for analysis. MS1 Mode can be used to view the purity of the synthesized or separated products (cinnamic acid: m/z 147, caffeine: m/z 195), and further identification of the compounds can be carried out using tandem mass spectrometry (cinnamic acid: MS2 m/z 119; caffeine: MS2 m/z 138). Each student can immediately characterize their own samples after the experiment and obtain data for their experimental report.
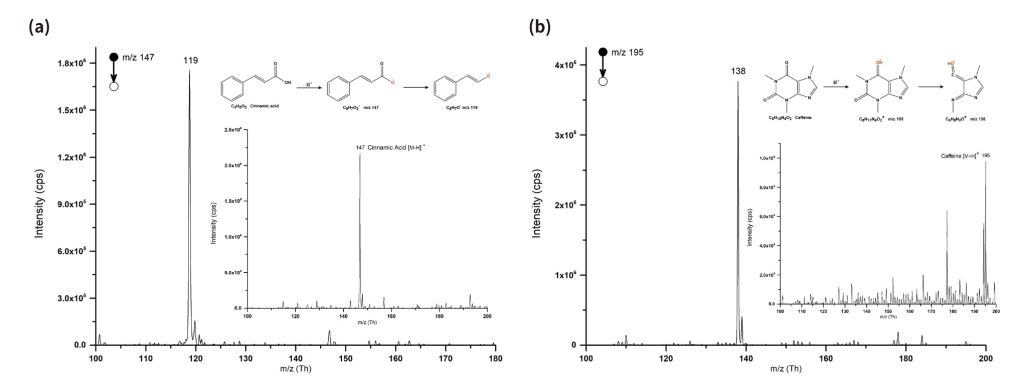
Figure 2. MS & MS/MS of cinnamic acid and caffeine (a) MS & MS/MS of cinnamic acid. (b) MS & MS/MS of caffeine.