Next-Generation Paternò–Büchi Reagents for Lipid Analysis by Mass Spectrometry
The Paternò–Büchi (PB) reaction is a photochemical reaction involving [2 + 2] cycloaddition between electronically excited carbonyl and carbon–carbon double bond (C=C). It has been established as a lipid derivatization strategy, leading to confident assignment of C=C locations in lipids when coupled with tandem mass spectrometry (MS/MS). Although acetone and several aryl-containing ketones or aldehydes have been explored as PB reagents, the chemical properties critical to achieving efficient conversion and minimum side reactions remain unclear. Herein, we investigated a set of acetophenone (AP) derivatives, aiming to provide insights into the development of new PB reagents with enhanced performance for lipid analysis. For AP derivatives, we found that electron-withdrawing groups (e.g., −F and −CF3) on the benzene ring improved the overall conversion, while a bulky group at the ortho-position decreased the conversion. Norrish Type I cleavage was largely diminished; however, the Norrish Type II side reaction was more competitive, producing products isomeric to the PB reaction products. Among all AP derivatives tested, 2′,4′,6′-trifluoroacetophenone (triFAP) showed the best performance. It offered a relatively high PB yield (20–30%) for different types of C=C, high sensitivity (sub-nM) for C=C identification, and accurate isomer quantitation. Due to the significantly reduced chemical interferences in shotgun analysis, triFAP provided better performance than that from acetone PB-MS/MS. An offline triFAP PB reaction was implemented in a liquid chromatography analysis workflow, which enabled the large-scale identification of phospholipids including C=C location isomers from a complex lipid extract.
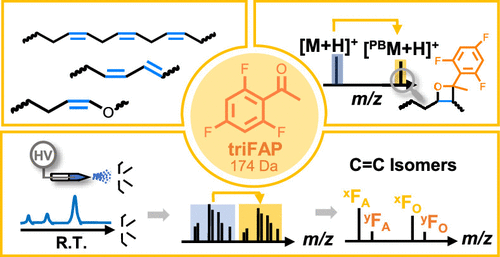
- This study systematically evaluates acetophenone derivatives to disclose the key chemical features that influence the efficiency of Paternò-Büchi (PB) reaction and side reactions for lipid C=C derivatization.
- It determines 2′,4′,6′-trifluoroacetophenone (triFAP) as the optimal PB reagent, providing high C=C derivatization yields, sub-nanomolar sensitivity, and improved quantification of isomers.
- The triFAP-based PB derivatization is then incorporated into liquid chromatography-tandem mass spectrometry, enabling large-scale identification of phospholipid C=C location isomers with minimal chemical interference.
Anal Chem. 2020 Oct 6;92(19):13470-13477. (IF: 6.7)


